16 Beauty Products from the Past That Wouldn’t Get FDA Approval Today
Several beauty products from the past contained harmful ingredients or made unsafe claims that would not meet today’s FDA standards.
- Sophia Zapanta
- 5 min read

In earlier decades, the beauty industry was less regulated, allowing the sale of products with toxic chemicals or misleading promises. Many items included mercury, lead, or radioactive elements, posing serious health risks. If released today, these products would be rejected under modern FDA guidelines for safety and labeling.
1. Lash Lure Eyelash Dye
 The U.S. Food and Drug Administration on Wikimedia Commons
The U.S. Food and Drug Administration on Wikimedia Commons
Lash Lure was an eyelash and eyebrow dye sold in the 1930s. It contained para-phenylenediamine, a chemical that caused blindness and even death. The product was linked to severe allergic reactions. It helped lead to stronger cosmetic regulations in the U.S.
2. Radium-Based Face Creams
 cosmesi fai da te naturale on Wikimedia Commons
cosmesi fai da te naturale on Wikimedia Commons
Some early 20th-century creams claimed to “revive” the skin using radium. These radioactive products were marketed as anti-aging solutions. They caused burns, illness, and long-term radiation damage. The FDA would immediately ban them today due to radiation exposure.
3. Lead-Based Face Powders
 Jen on Wikimedia Commons
Jen on Wikimedia Commons
Face powders used during the 18th and 19th centuries often contained white lead. These powders gave a pale look that was considered fashionable. Prolonged use led to skin deterioration, headaches, and lead poisoning. Modern rules prohibit toxic heavy metals in cosmetics.
4. Tho-Radia Skin Cream
 stux on Wikimedia Commons
stux on Wikimedia Commons
This French beauty cream was sold in the 1930s and included both thorium and radium. It was advertised as a scientific skincare breakthrough. Users were exposed to radiation with every application. Today, radioactive materials are banned from all consumer products.
5. Mercury Skin Lightening Creams
 Demar thomas on Wikimedia Commons
Demar thomas on Wikimedia Commons
Some vintage creams used mercury compounds to lighten skin. Mercury is highly toxic and can damage the kidneys and nervous system. It was absorbed through the skin and accumulated in the body over time. The FDA now restricts mercury use to tiny amounts in eye products only, under strict conditions.
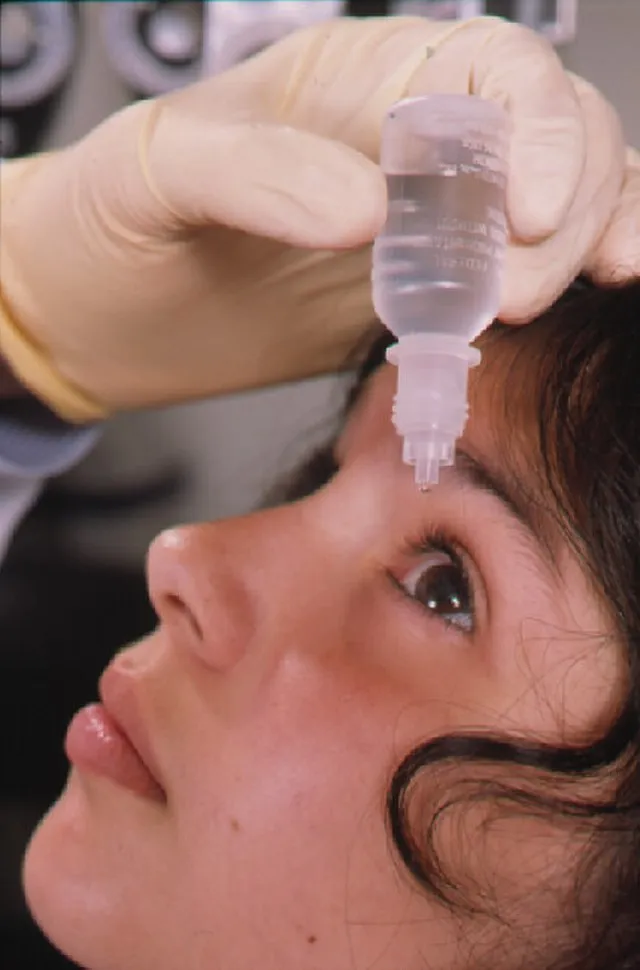
6. Belladonna Eye Drops
 National Eye Institute on Wikimedia Commons
National Eye Institute on Wikimedia Commons
Belladonna drops were used to dilate pupils and make eyes appear larger and more attractive. The substance is derived from a toxic plant that affects the nervous system. Prolonged use could lead to vision issues and poisoning. These drops are no longer approved for cosmetic use.
7. Carbon Tetrachloride in Hair Products
 Pittigrilli on Wikimedia Commons
Pittigrilli on Wikimedia Commons
Used in some early hair products as a cleaning solvent, carbon tetrachloride is a toxic chemical. It can harm the liver, kidneys, and central nervous system. Inhalation or skin contact posed health risks for users and salon workers. It’s banned in cosmetic products today.
8. Hazel Atlas Uranium Glass Lipstick Holders
 Joe Haupt on Wikimedia Commons
Joe Haupt on Wikimedia Commons
Lipsticks in the 1930s and 1940s were sometimes packaged in uranium glass holders. These glowed under UV light and were marketed as luxury items. The uranium content emitted low-level radiation. Modern FDA and consumer safety rules do not allow radioactive packaging.
9. Antimony Eyeliner (Kohl)
 Yugaljoshi on Wikimedia Commons
Yugaljoshi on Wikimedia Commons
Traditional kohl used in some cultures contained antimony and lead. These heavy metals posed long-term health risks, including lead poisoning. The FDA prohibits the import and sale of kohl containing these ingredients. Only safe, regulated versions are allowed on the market today.
10. Formaldehyde-Based Nail Hardeners
 cottonbro studio on Pexels
cottonbro studio on Pexels
Some vintage nail hardeners relied on formaldehyde to strengthen nails. Formaldehyde is a known irritant and potential carcinogen. It caused skin reactions and respiratory issues for some users. Today, formaldehyde use is restricted in personal care products.
11. Chloroform in Perfumes
 DDupard on Wikimedia Commons
DDupard on Wikimedia Commons
Chloroform was once used to stabilize the scent in certain perfumes. It has since been linked to liver damage and cancer. Inhalation posed a health risk over time. It is no longer allowed in U.S. cosmetic formulations.
12. Dioxane in Bubble Baths
 Fæ on Wikimedia Commons
Fæ on Wikimedia Commons
Older bubble bath formulas often included dioxane as a byproduct of surfactants. Dioxane is a potential carcinogen and environmental toxin. It absorbs easily through the skin and has been found in unsafe levels in some past products. The FDA recommends that manufacturers remove it from cosmetics.
13. Coal Tar in Hair Dyes
 Mostafameraji on Wikimedia Commons
Mostafameraji on Wikimedia Commons
Coal tar was a common ingredient in early hair dyes. It has been linked to skin irritation and cancer risks. The FDA now requires clear warnings or bans its use in many products. Safer synthetic dyes have since replaced it.
14. Bithionol in Soaps and Creams
 Kritzolina on Wikimedia Commons
Kritzolina on Wikimedia Commons
Bithionol was once used as an antibacterial agent in soaps and creams. It caused extreme sensitivity to sunlight, leading to rashes and skin damage. The FDA banned it after discovering its harmful side effects. It’s no longer permitted in any over-the-counter cosmetics.
15. Talc Contaminated with Asbestos
 McZusatz on Wikimedia Commons
McZusatz on Wikimedia Commons
Some past talcum powders were found to contain asbestos, a known carcinogen. The contamination happened during mining, where talc and asbestos naturally occur together. Inhalation over time increased the risk of lung disease and cancer. The FDA now requires strict testing for talc-based products.
16. Synthetic Estrogens in Anti-Aging Creams
 Harry Klenda on Wikimedia Commons
Harry Klenda on Wikimedia Commons
Some vintage anti-aging creams used synthetic estrogens to improve skin elasticity. These hormones disrupted endocrine function and raised cancer risks with long-term use. The FDA regulates any hormone-related compounds strictly. These products would not be allowed without proven safety and approval.